Your Location:Home >Products >Fine chemicals >625-05-8
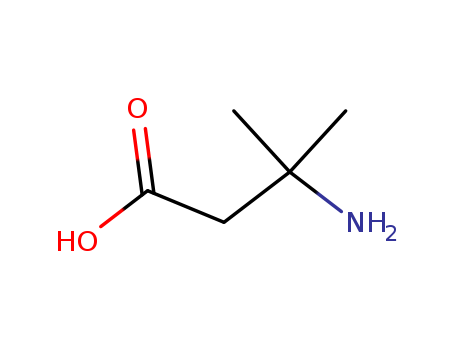

Product Details
|
Uses |
3-Amino-3-methylbutanoic Acid acts as a reagent in the preparation of arylsulfonyl methylbutanamides of cycloalkylamines, particularly adamantylamines, as selective inhibitors of human and murine 11β-hydroxysteroid dehydrogenase type 1. Synthesis of acetylcholine and carbamoylcholine analogs as a functionally selective α4β2 nicotinic acetylcholine receptor agonist. Phenamide preparation by coupling reaction. |
InChI:InChI=1/C5H11NO2/c1-5(2,6)3-4(7)8/h3,6H2,1-2H3,(H,7,8)
A versatile methodology for the preparat...
2-Dichloroamino-2-methyl-propane-1-sulfo...
A series of backbone modified and sulfon...
N-Methyl β-amino acids are generally req...

3-benzylamino-3,3-dimethylpropionic acid


3-amino-3-methylbutanoic acid
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; hydrogen; acetic acid; 5%-palladium/activated carbon; In ethanol; water; at 50 ℃; for 5h;
|
100% |

3-Methylbutenoic acid


3-amino-3-methylbutanoic acid
| Conditions | Yield |
|---|---|
|
With ammonium hydroxide; In water; at 150 ℃; for 16h; under 36200.4 Torr;
|
42% |
|
With ammonia; water; at 150 ℃; ueber mehrere Stufen;
|
|
|
Multi-step reaction with 2 steps
1.1: ethane-1,2-diol / 1 h / 190 °C
2.1: aq. NaOH
2.2: aq. HCl
With sodium hydroxide; In ethylene glycol; 1.1: Michael addition;
|
|
|
Multi-step reaction with 2 steps
1: ethane-1,2-diol / 1 h / 190 °C
2: NaOH / H2O
With sodium hydroxide; In water; ethylene glycol;
|
|
|
Multi-step reaction with 2 steps
1: sodium azide; acetic acid / 48 h / 95 °C
2: palladium; hydrogen / ethyl acetate / 24 h / 20 °C
With sodium azide; hydrogen; palladium; acetic acid; In ethyl acetate;
|

3-Methylbutenoic acid

3-methyl-3-nitrobutanoic acid

4,4-dimethyl-azetidin-2-one

Sodium; 3-amino-3-methyl-butyrate

3-benzoylamino-3-methylbutanoic acid

β-glycylamino-isovaleric acid

C11H27NO2Si2

N-<2-Hydroxy-1.3-dioxo-2.3-dihydro-phenalenyl-(2)>-β-aminoisovaleriansaeure
CAS:2941-78-8
CAS:1143516-05-5
CAS:74784-70-6
CAS:2935-90-2